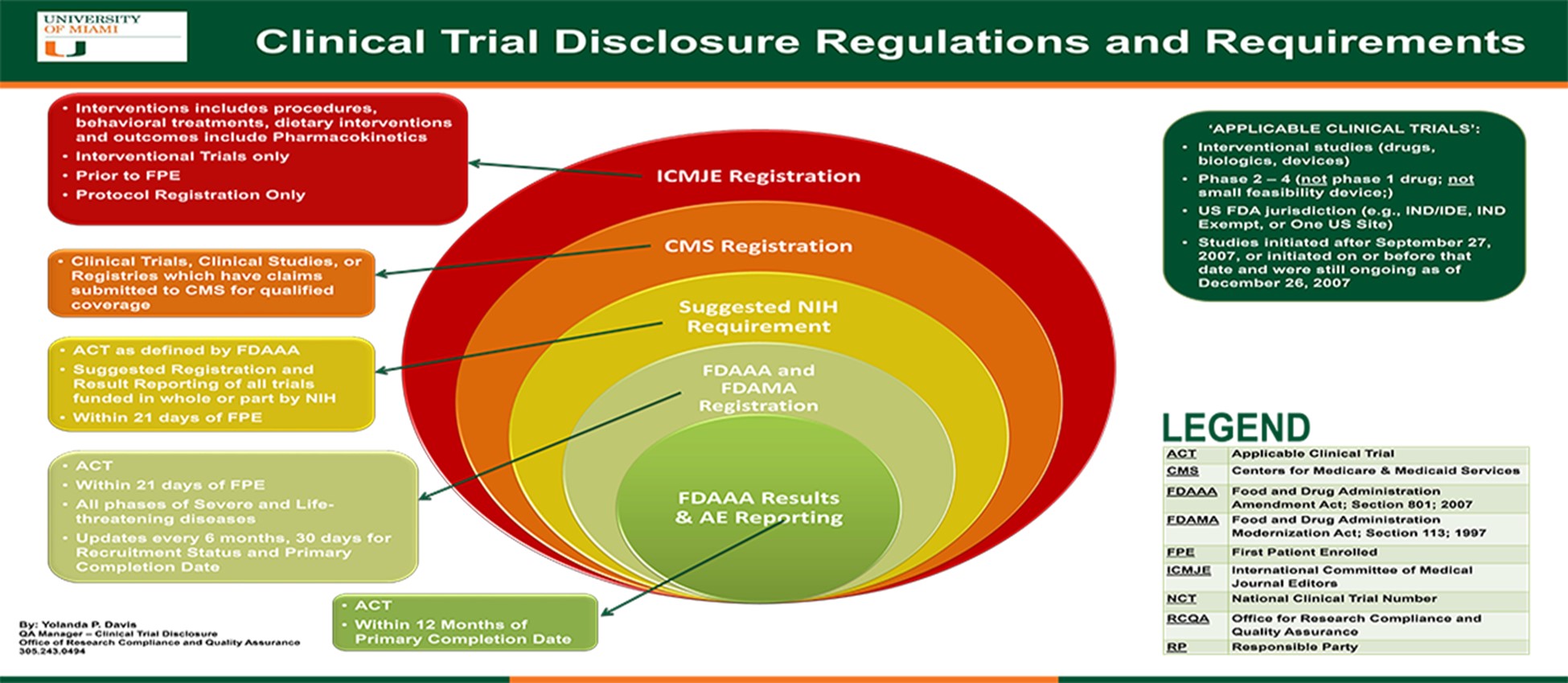
The Food and Drug Administration (FDA), the National Institutes of Health (NIH), Centers for Medicaid and Medicare Services (CMS), and the International Committee of Medical Journal Editors (ICMJE), all have adopted regulations and/or requirements surrounding the registration and result reporting of clinical trials on the ClinicalTrials.gov website.
Not complying with any of these regulations and/or the University of Miami policy may result in the following: inability to publish; civil monetary penalties levied against the University; suspension of protocol approval from the IRB; loss of additional or continued funding from federal agencies and other entities or inadequate reimbursement.
Notice of Non-Compliance
Please contact the Clinical Trial Disclosure group at ctgovum@miami.edu or (305) 243-1107 if you are contacted by any agency, institution or group regarding lack of registration or result reporting.