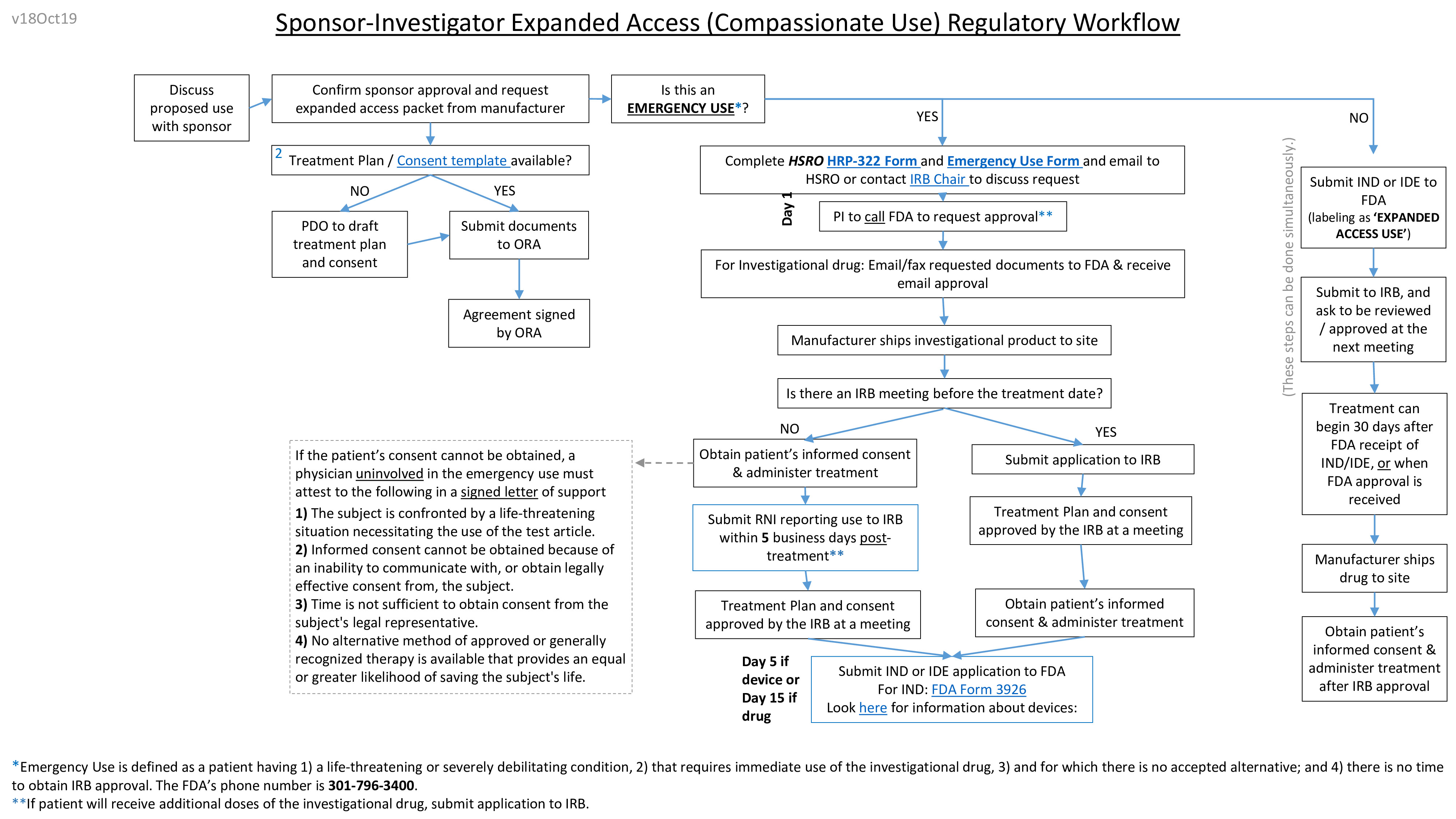
“Emergency Use” is a provision of the FDA regulations that allows a physician to use an investigational product in a life-threatening emergency when there isn’t sufficient time to obtain IRB review and approval. Physicians must complete the following seven steps.
Step One
When you decide that the investigational product is the best option for the patient in an emergency, contact the FDA to obtain approval.
- For an investigational drug or biologic call (888) 463-6332 or email druginfor@fda.hhs.gov
- For an investigational device call (301) 796-7100 or email dice@fda.hhs.gov.
Step Two
The next step is to contact the sponsor and obtain agreement for the use of the investigational product.
Step Three
Check to see if the regulatory criteria for use of the investigational product without IRB approval are met by reviewing Worksheet Emergency Use (HRP-322).. If time permits, complete and submit an Emergency Use Request Form and Worksheet Emergency Use (HRP-322) to hsro@miami.edu and call the HSRO at 305-243-3195. During evenings or weekends you can call one of the IRB Chairs to discuss the emergency use.
Step Four
If time permits, you must obtain informed consent from the patient using TEMPLATE CONSENT DOCUMENT – Emergency Use (HRP 502h). If the patient is not able to provide informed consent due to incapacity and there is insufficient time to obtain consent from a legally authorized representative, see the "Exception from the Requirement for Informed Consent for Emergency Use" in the following group.
Step Five
You must report the use of the unapproved drug, biologic or device to the IRB through an eProst submission within five business days using the electronic Report of New Information SmartForm in eProst. Regulations require that you submit this report even when you discussed the use of the investigational product with the sponsor and IRB Chair. If you fail to submit the report within five days, you will be restricted from submitting new Human Research until the IRB receives this report and your action plan to ensure you submit timely emergency use reports in the future.
Step Six
Complete and submit the IND or IDE application to the FDA. For IND, use FDA Form 3926. For IDE, look here for information:
Step Seven
Finally, according to the FDA, the emergency use provision can be used only once. If there is any possibility that you will need to use the same investigational product with the same patient or with a different patient, you must submit a protocol to the IRB and obtain an IND or IDE from the FDA. The FDA regulations have provisions for expanded access use through a treatment protocol. See
FDA Guidance on Expanded Access for additional information.