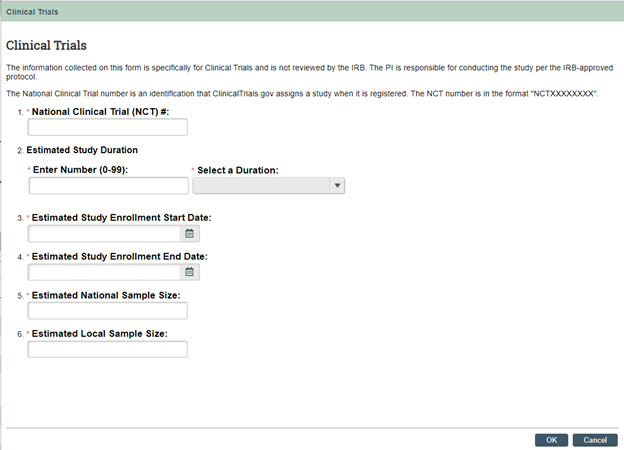
Investigator Manual Update
An updated version of the HSRO Investigator Manual is now available on the HSRO Website. A copy canbe found HERE
Description of Changes
Significant revisions:
· BSN prepared nurses may serve as a PI when they meet additional requirements.
· Community Partners may take the training, Community Involvement in Research Training Program – Training for Community Partners instead of the CITI training.
· We added sections on simplifying consent language, and remote consent to the chapter on Informed Consent
· The HSRO extended the reporting time frame for new risk information from five days to 10 days.
· We added a requirement to list the location where the non-compliance occurred on RNI reports of non-compliance so the HSRO can comply with the responsibility to inform Jackson Health Systems of non-compliance that arises at a Jackson Health System facility.
· We made significant revisions to the section on Emergency Use of an Investigational Product. The HSRO now requires the physician to submit their five-day post-use report on the HSRO’s form, Emergency Use Report.
· We advised investigators that the IRB will apply ICH-E6(r2) (ICH) requirements when the protocol calls for this compliance. However, the HSRO will not routinely enforce the following:
o The requirement to list risks of alternative treatments in the consent document; and
o The requirement to inform the participant’s regular physician (PCP) about that individual’s enrollment in a clinical trial when the participant agrees (The HSRO recommends that PIs adhere to this requirement when informing the PCP is needed to reduce the risk to the subject. However, in some instances, informing the participant doesn’t have a PCP, the information, the study involves sensitive information, or the disclosure is not feasible).
Other revisions:
The HSRO added:
· The Bio-Safety training requirements to the section on training;
· The requirement to document the study status in the Modification SnartForm;
· Emphasis on the requirement for HSRO sign-off before you start a study using an external IRB for oversight;
· A note that the HSRO does not require redline versions of changes to documents for studies using an external IRB for oversight.
· A requirement that the PI obtain the continuing review report and a preventive action plan from a relying site that has not submitted a timely continuing review report;
· New reminders to the chapter on Recruiting Study Participants:
o FDA Regulations prohibit representing an investigational product as safe and effective for the use under investigation in a clinical trial;
o Do not submit case report forms or data collections forms (except for retrospective chart review); and
o When the study involves a retrospective record review, the HSRO needs a list of the data elements you plan to collect/review.
Administrative changes
· We simplified the language.
· We added a link to the Table of Contents (TOC) at the bottom of every page to simplify navigation.
· We updated the Table of Contents (TOC) was updated.
· We updated the links to external documents to ease navigation.
Please read this updated manual to ensure you are aware of the HSRO’s requirements and guidance.
Thank you!
Cindy
Cynthia Gates, JD, ADN, CIP
Executive Director, Human Subject Research Office
Office of Vice Provost for Research & Scholarship
University of Miami